_1711527830.png)
Lorlanib-100
Product Code: 0
Pack Size: 30
- Price: $763.636 ৳93208 ,
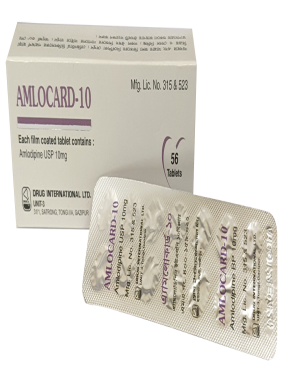
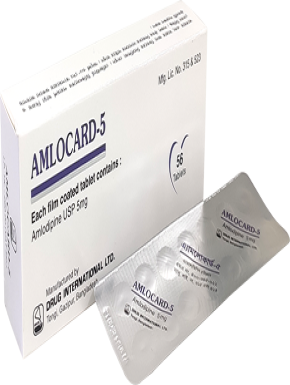
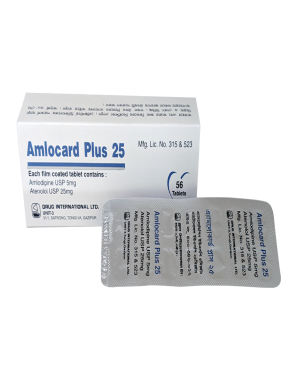
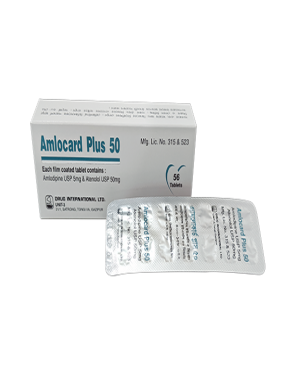
Each film coated tablet contains Lorlatinib INN 100mg.
It is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic Non-small Cell Lung Cancer (NSCLC) whose disease has progressed on • Crizotinib and at least one other ALK inhibitor for metastatic disease; or • Alectinib as the first ALK inhibitor therapy for metastatic disease; or • Ceritinib as the first ALK inhibitor therapy for metastatic disease
Major Ingredients
Each film coated tablet contains Lorlatinib INN 100mg.
Major Functionality
It is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic Non-small Cell Lung Cancer (NSCLC) whose disease has progressed on • Crizotinib and at least one other ALK inhibitor for metastatic disease; or • Alectinib as the first ALK inhibitor therapy for metastatic disease; or • Ceritinib as the first ALK inhibitor therapy for metastatic disease
Manufacture Information
Drug International Limited was incorporated under the Registrar of Joint Stock Companies in 1974 as a Private Limited Company. The company commenced formulation and production in 1983 and emerged as a pioneer in Bangladesh for adding a state of the art oral solid dosage plant. Since inception, Drug International Limited's primary objective has been to meet guidelines provided by major global regulatory bodies such as the World Health Organization Good Manufacturing Practices (WHO cGMP) guidelines.



_1711532236.png)
_1711528829.png)





_1694323949.png)
_1694323769.png)
_1694324979.png)