MUKOF
Product Code: M-00092
Pack Size: 30's
- Price: $3.97 ৳337.33 ,
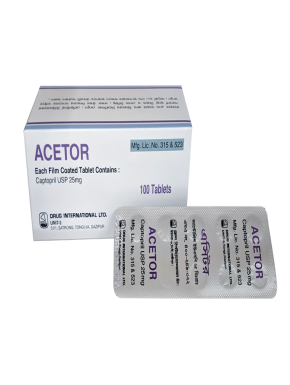
Each Dispersible Tablet Contains Acetylcysteine USP 600mg.
It is indicated as an adjunctive treatment for patients with abnormal, viscid or inspissated mucus secretions associated with conditions such as · Acute and chronic bronchopulmonary disorders (e.g. pneumonia, bronchitis, emphysema, tracheobronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis, primary amyloidosis of the lung) · Atelectasis caused by mucus obstruction · Pulmonary complications of cystic fibrosis · Pulmonary complications of thoracic and cardiovascular surgery & · Post-traumatic chest conditions. It is effective in all respiratory airways disease causing formation of a dense secretion that cannot be or can only partially be expectorated such as acute and chronic bronchitis, laryngitis, sinusitis, tracheitis, influenza & bronchial asthma. Acetylcysteine is also indicated in the treatment of Paracetamol overdose. Treatment option is optimal if given within 8 hours of Paracetamol ingestion.
Major Ingredients
Each Dispersible Tablet Contains Acetylcysteine USP 600mg.
Major Functionality
It is indicated as an adjunctive treatment for patients with abnormal, viscid or inspissated mucus secretions associated with conditions such as · Acute and chronic bronchopulmonary disorders (e.g. pneumonia, bronchitis, emphysema, tracheobronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis, primary amyloidosis of the lung) · Atelectasis caused by mucus obstruction · Pulmonary complications of cystic fibrosis · Pulmonary complications of thoracic and cardiovascular surgery & · Post-traumatic chest conditions. It is effective in all respiratory airways disease causing formation of a dense secretion that cannot be or can only partially be expectorated such as acute and chronic bronchitis, laryngitis, sinusitis, tracheitis, influenza & bronchial asthma. Acetylcysteine is also indicated in the treatment of Paracetamol overdose. Treatment option is optimal if given within 8 hours of Paracetamol ingestion.
Manufacture Information
Drug International Limited was incorporated under the Registrar of Joint Stock Companies in 1974 as a Private Limited Company. The company commenced formulation and production in 1983 and emerged as a pioneer in Bangladesh for adding a state of the art oral solid dosage plant. Since inception, Drug International Limited's primary objective has been to meet guidelines provided by major global regulatory bodies such as the World Health Organization Good Manufacturing Practices (WHO cGMP) guidelines.


_1711527830.png)

_1711532236.png)
_1711528829.png)





_1694323949.png)
_1694323769.png)
_1694324979.png)