
NAPSEC 375
Product Code: M-00112
Pack Size: 30's
- Price: $2.13 ৳181.04 ,
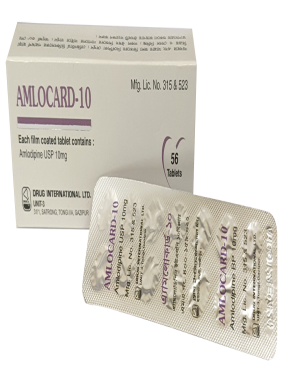
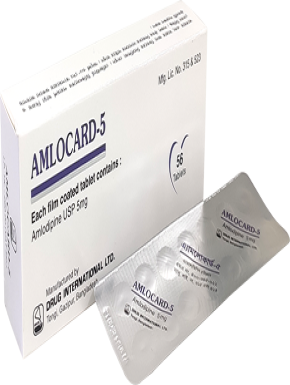
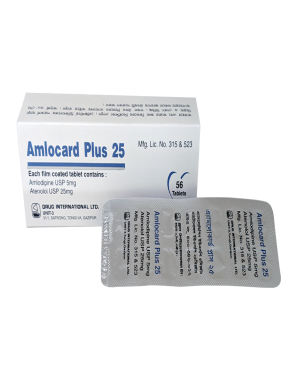
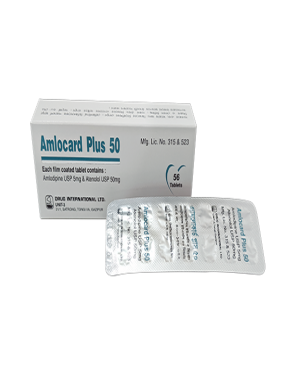
Naproxen BP 325 mg + Esomeprazole USP 20 mg Enteric Coated Tablet.
This is indicated for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, dysmenorrhoea and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers.
Major Ingredients
Naproxen BP 325 mg + Esomeprazole USP 20 mg Enteric Coated Tablet.
Major Functionality
This is indicated for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, dysmenorrhoea and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers.
Manufacture Information
Drug International Limited was incorporated under the Registrar of Joint Stock Companies in 1974 as a Private Limited Company. The company commenced formulation and production in 1983 and emerged as a pioneer in Bangladesh for adding a state of the art oral solid dosage plant. Since inception, Drug International Limited's primary objective has been to meet guidelines provided by major global regulatory bodies such as the World Health Organization Good Manufacturing Practices (WHO cGMP) guidelines.


_1711527830.png)

_1711532236.png)
_1711528829.png)





_1694323949.png)
_1694323769.png)
_1694324979.png)