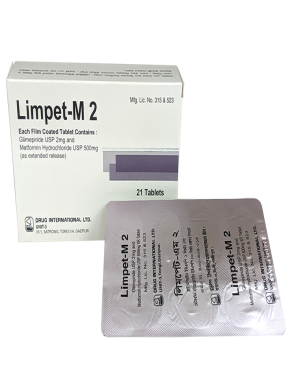
LIMPET M 2
Product Code: M-00064
Pack Size: 21's
- Price: $2.22 ৳188.91 ,
Each Film Coated Tablet Contains Glimepiride USP 2 mg and Metformin Hydrochloride USP 500 mg as Extended Release.
This combination is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both Glimepiride and Metformin is appropriate or in cases where Glimepiride/Metformin mono-therapy does not result in adequate glycemic control.
Major Ingredients
Each Film Coated Tablet Contains Glimepiride USP 2 mg and Metformin Hydrochloride USP 500 mg as Extended Release.
Major Functionality
This combination is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both Glimepiride and Metformin is appropriate or in cases where Glimepiride/Metformin mono-therapy does not result in adequate glycemic control.
Manufacture Information
Drug International Limited was incorporated under the Registrar of Joint Stock Companies in 1974 as a Private Limited Company. The company commenced formulation and production in 1983 and emerged as a pioneer in Bangladesh for adding a state of the art oral solid dosage plant. Since inception, Drug International Limited's primary objective has been to meet guidelines provided by major global regulatory bodies such as the World Health Organization Good Manufacturing Practices (WHO cGMP) guidelines.


_1711527830.png)

_1711532236.png)
_1711528829.png)





_1694323949.png)
_1694323769.png)
_1694324979.png)